



The main causes of acid rain are
•Volcanic activity
•A burned fossil fuel, that is, smoke from factories and auto emissions from diesel cars making
a nitrogenous from fertilizer derived nitrogen oxide (NOx),
It is found in car exhaust emission.
It bring about oxidase smog and acid rain.
Nitric monoxide (N₂O) allow for greenhouse effect and ozone depletion.
Reaction water and oxygen in atmosphere make acid strength like
a sulfuric acid, nitric acid, and hydrochloric acid, it become stronger acidly, it rains in our town.
It become stronger acidly, it rains in our town.
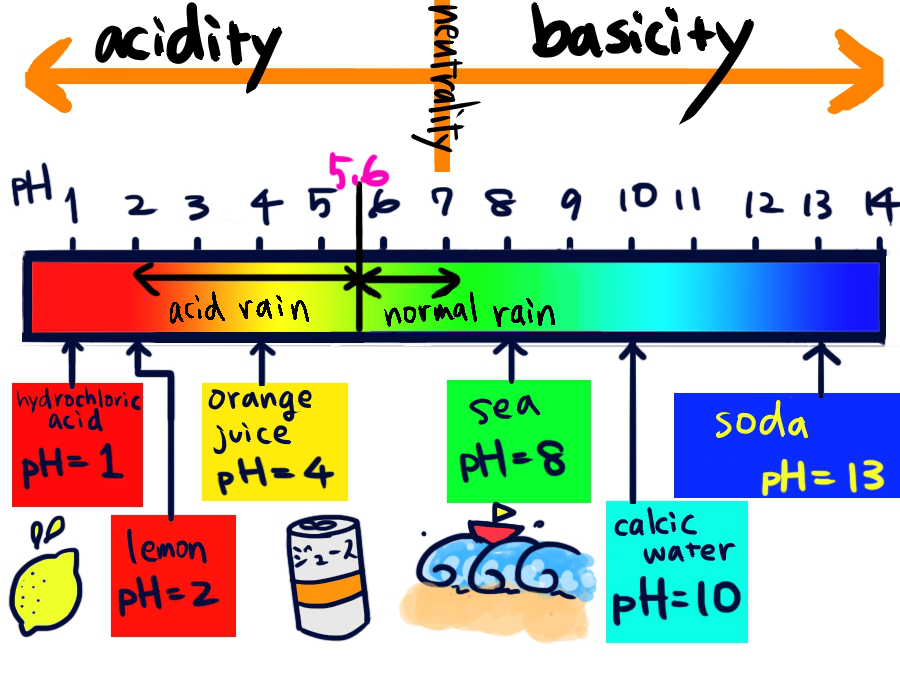
In fact, a pH
Hydrogen ion (H+) content in water solution.
If it is one atmosphere, 25℃ and pH=7, it get called in neutrality.
The more pH is low, acidity is strong.
And the more pH is high, acidity is weak.
power of Hydrogen. under 7 is considered acidic.
But acid rain is under pH 5.6, because CO2
Gas which is show low acidity.
It generated by burning of material that contains carbon or activity of a volcano.
Substance responsible for rain is mild acidity from the first.
in the atmosphere is dissolved in rain.
Severe smoke screens and smog
Aerosol layer which caused by large consumption of coal and oil.
Alternatively condition have poor visibility
because air contaminant is floating on air. occurs close to factories
in European and Eastern American neighborhoods.
Chimneys have been made taller in order to create lower concentrations of acid rain.
This is a more in-depth explanation of acid rain
Incidentally, 'smog' is a combination of the words 'smoke' and 'fog'.