iPS cells
iPS research advanced as a new possibility of altering embryonic stem cells.
First of all, we will explain the outline of iPS cells.
iPS cells refer to cells which have pluripotency equivalent to embryonic stem cells and are made from the Somatic stem cells which are obtained from the patient's own organs.
iPS cells have fewer risks of ethical problems and rejection, because it is made from the somatic stem cells which are obtained from the patient's own organs.
However, what becomes a problem here, is the pluripotency of the body's cells have been lost.
As we explained in the section about differentiation and outbreak, advances in this specialization are usually one-way.
The the way of thinking that the outbreak fate that had been settled could not change was common.
Yet an occurrence to overturn this way of thinking took place.
The cloned sheep Dolly ü`possibility of initializing cellsü`
That occurrence is the birth of the cloned sheep "Dolly" which was announced in 1997. (Birth of "Dolly" is 1996.)
I think you have heard the phrases "clone" at least 1 time.
Although there are various methods in the creation of a clone, we will explain the cloning procedure of turning somatic cells into clones like Dolly briefly.
First, we prepare the unfertilized egg of the normal sheep and remove only the nucleus from it.
After having done that, we transplant the somatic cell which we took out and add special electrical stimulation and return it to the uterus afterwards after having induced cell division.
Dolly could not be born if the outbreak fate ofü@somatic cells was not changed.
This opinion is reversed by Dolly's birth, and it has proved that such cells can be initialized.
It has proved about the initialization of cells in this way, but originary did not lead to the identification of the factor necessary for initialization in those days.
Therefore, the inventor of iPS cells professor Shinya Yamanaka of the Kyoto University iPS cell research center built the hypothesis, "What" is required in order to make it change to the cell which initializes other cells and has pluripotency will be the same as a factor required for an embryonic stem cell to demonstrate pluripotency.
Applied ES cell's technologyü`birth of iPS cellsü`
It is very hard if we have to analyzeü@the gene of an embryonic stem cells exhaustively.
Fortunately, studies which analyze genes functioning with every cell in mice and compile into a database are becoming more common in Japan.
They judged that the cancer-related genes which controll the levels of the cells and the gene which has outstanding activity in the embryonic stem cells would be found by refering to the released genes' data.
They started a study to decide a candidate cell by investigating the database.
As a result, the factors which are considered to be indispensable for maintaining pluripotency were narrowed down to 24 in 2004.
Then, they made the cell which removed one among the 24 factors each and they carried out experiment.
To pinpoint which their factors demanding are in 24 factors
Then there are ten cells which are abnormally produced.
From the results, the factor in connection with maintaining pluripotency was narrowed down to ten pieces.
They went through the same procedure for the next ten factors and they succeeded in narrowing down the factor to four genes.
To narrow it down more precisely, they changed the technique of the experiment and made the following cells.
ć@The cells which put in all four factors
ćAThe cells which put in only one of four factors
ćBThe cells which put in two of four factors
ćCThe cells which eliminated only one of four factors
The state that keeps all four factors is the best in the four above-mentioned cells.
Meanwhile, they confirmed that if at least one lacks a factor, a function will fall, and only in two factors, there is not activity at all.
In short, these four factors are not allowed to be missing even one.
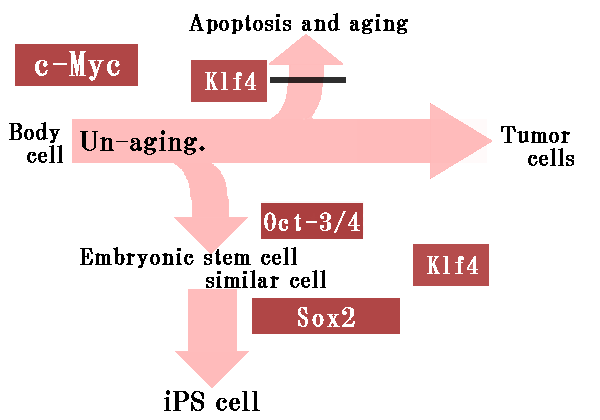
Regarding completed cells, they conductedü@a comprehensive analysis about what kind of gene is functioning.
As a result of such an analysis, it was found that the obtained cells have the character of embryonic stem cells instead of the character of cells in which the original pluripotency was lost.
Finally, they transplanted it under the skin of a mouse in order to study what kind of motion this cell carries out in the body of the animal.
As a result of such experiments, it formed a teratoma with features, such as nerve, cartilage and epithelial cells, muscles, and an alimentary canal. Thereby, it was confirmed that a cell had pluripotency.
Moreover, as result of culturing this cell in nutritive liquid, and observing it, they found out that the cells have the ability to differentiate to the cells of various organs.
At last, they succeeded in clarifying the factor which initializes the cells which have lost pluripotency and regain pluripotency again by this series of studies.
After this, they succeeded in the establishment of the induced pluripotent stem cells with the human cell and an article was announced in November, 2007 .
iPS cells of the future
In this way, iPS cells were born. However,their mechanism yet was not completely elucidated, and the expected result is not given about the efficiency of cultivation, etc.
If the elucidation of ips cells advances,the infertility treatment using organ transplantation and iPS cells derived from ips cells can be put to practical use in the near future. Therefore, iPS cells could become a new light to explore in the future.
The Ministry of Education, Culture, Sports, Science and Technology made a road map about the concrete practical use and study of iPS cells in 2009.(November, 2012 revision edition)
The table below summarizes the contents of the road map.
| name | The clinical experiment start year (data of 2009) | Change by the November, 2012 revision |
| Central nervous system | 2015 | none |
| Cornea | 2015 | 2-4 years postponement |
| Retinal pigment epithelial cells | 2011 | none |
| Visual cells | 2016 | none |
| Blood platelets | 2014 | none |
| Red corpuscles | 2019 | moving it up until around 2017 |
| Hematopoietic stem cell | 2016 | 3-6 years postponement |
| Cardiac muscle | 2014 | none |
| A bone and a cartilage | 2019 | none |
| Skeletal muscle | 2019 | none |
| Entoderm system cell | 2019 | none |
| The iPS cells bank for regenerative medicine | It aims at building by 2014 | It aims at building by the end of 2013 |
āzü[āĆāyü[āW āeāōāvāīü[āg ātāŖü[
Design by

